Study on epidemic factors of varicella outbreak under high varicella vaccine coverage
-
摘要:
目的 探索1剂次水痘疫苗高覆盖率下水痘暴发的流行因素及1剂次水痘疫苗的保护效果,为控制水痘暴发疫情、调整优化水痘免疫策略提供科学依据。 方法 采用1:2配对病例对照研究,对2018年发生于江苏省苏中地区一中心小学的一起水痘暴发疫情开展流行病学调查。水痘暴发的流行因素分析采用条件Logistic逐步回归分析。 结果 该疫情共持续14 d,共报告水痘确诊病例45例,其中突破病例占71.1%。突破病例的发热情况、出疹程度、病程与无免疫史病例相比均较轻(均有P < 0.05)。条件Logistic回归分析提示,参加课外辅导机构(OR=2.6,95% CI:2.0~3.2),有兄弟姐妹(OR=2.5,95% CI:2.1~4.3),无水痘疫苗接种史(OR=2.7,95% CI:2.4~4.2),与水痘患者接触史(OR=2.4,95% CI:1.1~5.3)是水痘暴发的流行因素。接种年限>5年,初次免疫年龄 < 15月龄是发生突破病例的潜在危险因素(均有P < 0.05)。1剂次水痘疫苗的总体保护效果为77.9%(95% CI:53.3%~92.1%)。 结论 突破病例临床症状较轻,1剂次水痘疫苗不足以控制水痘暴发疫情,其疫苗保护效果有限。建议采用2剂次水痘免疫策略。 Abstract:Objective To explore the epidemic factors of varicella transmission under high varicella vacince coverage, assess the vaccine effectineness of one dose of varicella vaccine, so as to provide scientific basis for controlling the varicella outbreak and optimizing the varicella immunization strategy. Methods A 1:2 paired case-control study of a varicella outbreak was conducted in a primary school in central region of Jiangsu Province in 2018. Analysis of varicella epidemic factors was performed using conditional logistic stepwise regression. Results This outbreak lasted for 14 days. A total of 45 students were infected with varicella, of which 71.1% were breakthrough cases. The fever, rash degree and disease course of breakthrough cases were all relatively mild compared with those without immune history (all P < 0.05).The results of conditional logistic stepwise regression suggested that participating in extracurricular tutoring institutions(OR=2.6, 95% CI: 2.0-3.2), having brothers or sisters(OR=2.5, 95% CI: 2.1-4.3), without vaccination history of varicella vaccine(OR=2.7, 95% CI: 2.4-4.2), and contacting with varicella patients (OR=2.4, 95% CI: 1.1-5.3) were risks factor for varicella transmission. Time since vaccination >5 years and the initial immunization age < 15 months were potential risk factors for breakthrough cases. The overall vaccine effectiveness of one dose of varicella vaccine was 77.9%(95% CI: 53.3%-92.1%). The fever, severity of the rash and the course of the disease were all milder than those without the history of immunization (all P < 0.05). Conclusions The clinical symptoms of the breakthrough cases are relatively mild, and one dose of varicella vaccine is insufficient to control the outbreak of varicella with limited vaccine effectiveness. Two doses of varicella immunization strategy is recommended. -
Key words:
- Varicella vaccine /
- Vaccine effectiveness /
- Breakthough cases
-
水痘是由水痘带状疱疹病毒(varicella zoster virus, VZV)原发感染引起的高度传染性疾病,易在托幼机构、中小学等集体单位暴发流行[1]。目前,国家尚未将水痘疫苗纳入儿童免疫规划,但国内已有部分省市将水痘疫苗纳入免费接种项目。2018年江苏省水痘突发公共卫生事件数位于传染病相关事件数前列。水痘疫情已成为传染病防治及学校卫生工作的突出问题[2]。
本研究采用1 ∶ 2配对病例对照研究,对2018年6月发生在江苏省苏中地区某中心小学的一起水痘暴发疫情开展流行病学调查,为探究1剂次水痘疫苗的保护效果及水痘暴发的流行因素,以及调整优化水痘免疫策略提供科学依据。
1. 对象与方法
1.1 研究对象
突破性病例定义为至少接种1剂次水痘疫苗42 d后发生的水痘病例。病例纳入标准:(1)本次暴发(2018年6月1日之后)的新发临床诊断病例或实验室确诊病例;(2)本人及家属愿意配合调查;(3)就诊资料完整。排除标准:(1)拒绝流行病学调查;(2)同时患有其他疾病,可能干扰结果者。
采用1 ∶ 2匹配对照,其中1个对照在病例本班选取。另1个对照在其他未发生病例的班级选取。对照纳入标准:(1)既往无水痘患病史;(2)本人及家属愿意配合调查。对照排除标准:(1)既往有水痘患病史;(2)水痘疫苗免疫史不详。最后调查病例有45例,对照为90例。
1.2 调查内容
采用自行设计的问卷调查表,家长签署知情同意书并填写孩子个人信息问卷。疫苗免疫史以接种证为准;接种证缺失者的疫苗免疫史查询江苏省儿童预防接种信息管理系统进行核对。皮疹的严重程度定义:皮损<50个视为轻症,50~499个为中度出疹;皮损>500个视为重度出疹。并发症包括肺炎、脑炎、皮肤继发细菌感染等疾病。本研究已通过江苏省疾病预防控制中心伦理委员会批准。
1.3 疫苗效果评价指标
选用疫苗保护效果作为评价指标。疫苗保护效果(%)=(未接种组发病率-接种组发病率)/未接种组发病率×100%。当无法确切获得人群接种率时,使用病例对照研究,用OR来计算疫苗保护效果,因此,疫苗保护效果(%)=(1-OR)×100%。
1.4 统计分析
水痘传播因素分析采用条件Logistic回归分析。两组或多组率和构成比比较采用χ2检验或Fisher确切概率法。使用R 3.5.0软件进行统计分析,检验水准α=0.05。
2. 结果
2.1 暴发疫情概况
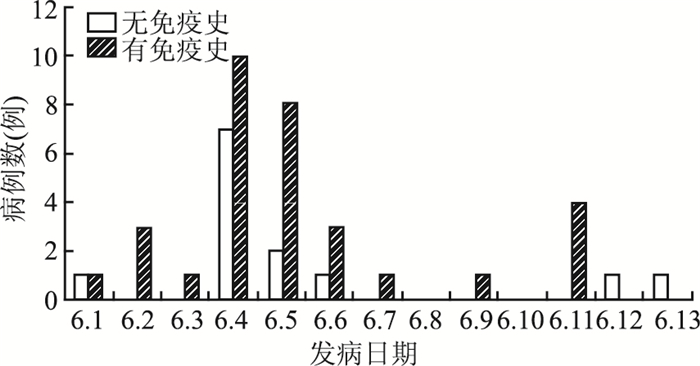
该起水痘暴发疫情持续时间为14 d,共有病例45例,均为临床诊断病例,罹患率为5.86%(45/768),其中突破病例有32例,占71.1%。调查发现的首发病例,男,11岁,三年级学生,于6月1日出现全身散在皮疹,以面部、躯干为主,伴瘙痒,6月2日到当地某县医院就诊,诊断为“水痘”。病例平均年龄为9.1(6.5~12.8)岁,男女发病比例为1.23 ∶ 1。所有学生均为本地户口。全校1剂次水痘疫苗覆盖率约为81.9%(629/768),不同免疫史患者发病时间分布见图 1。
2.2 突破病例与无免疫史病例的临床表现对比
突破病例重度出疹(皮疹>500个)的比例低于无免疫史病例,差异有统计学意义(χ2=7.79,P=0.020)。与无免疫史病例相比,突破病例病程、出疹部位数均较少,差异均有统计学意义(均有P<0.05)。见表 1。
表 1 突破病例与无免疫史病例的临床症状比较[n(%)]Table 1. Comparison of clinical symptoms between breakthrough cases and non-immune cases[n(%)]特征 突破病例 无免疫史病例 χ2值 P值 发热 5.47 0.019b 是 10(31.3) 9(69.2) 否 22(68.7) 4(30.8) 皮疹严重程度(个) 7.79 0.020b <50 25(78.1) 5(38.5) 50~ 6(18.8) 5(38.5) >500 1(3.1) 3(23.0) 并发症a 0.95 0.329 有 2(2.22) 2(6.7) 无 30(97.8) 11(93.3) 出疹部位(个) - 0.021b 1~ 14(43.8) 5(38.5) 3~ 16(50.0) 3(23.0) ≥5 2(6.2) 5(38.5) 病程(d) - <0.001b ≤10 23(73.3) 2(36.7) >10 9(26.7) 11(63.3) 注:a并发症包括肺炎、脑炎、皮肤继发细菌感染等疾病;bFisher确切概率法。 2.3 水痘流行因素分析
以性别、水痘疫苗免疫史、是否参加课外辅导机构、家庭人口构成、上学交通工具、与水痘病例接触史等指标作为自变量,以是否诊断为水痘病例作为因变量,进行单因素Logistic回归分析。结果显示,有兄弟姐妹(OR=2.3, 95% CI: 1.1~4.6)、参加课外辅导机构(OR=2.4, 95% CI: 1.1~4.9)、有与水痘患者接触史(OR=2.8, 95% CI: 1.6~5.9)、无水痘疫苗免疫史(OR=2.9, 95% CI: 2.1~4.8)是水痘暴发的流行因素。
采用逐步法对性别、是否有兄弟姐妹、是否参加课外辅导班、是否与水痘患者接触史、交通工具、水痘疫苗免疫史、洗手频率7个变量进行条件Logistic回归分析,纳入标准为P<0.10,剔除标准为P> 0.15。结果显示参加课外辅导班(OR=2.6, 95% CI: 1.9~3.2)、有兄弟姐妹(OR=2.5, 95% CI: 2.1~4.3)、有与水痘患者接触史(OR=2.4, 95% CI: 1.1~5.3)、无水痘疫苗免疫史(OR=2.7, 95% CI: 2.4~4.2)是水痘暴发的主要流行因素。见表 2。
表 2 水痘暴发的流行因素分析[n(%)]Table 2. Analysis of risk factors for Varicella transmission factors[n(%)]特征 病例 对照 P值 单因素分析OR (95% CI)值 P值a 条件Logistic回归OR(95% CI)值 性别 男 25(55.6) 49(54.4) 0.903 0.9 (0.5~1.9) 女 20(44.4) 41(35.6) 1.0 是否有兄弟姐妹 是 28(62.2) 38(42.2) 0.030 2.3 (1.1~4.6) 0.037 2.5 (2.1~4.3) 否 17(37.8) 52(57.8) 1.0 1.0 参加课外辅导机构 是 29(64.4) 39(43.3) 0.021 2.4 (1.1~4.9) 0.019 2.6 (1.9~3.2) 否 16(35.6) 51(56.7) 1.0 1.0 与水痘患者接触史 有 32(71.1) 42(46.7) 0.029 2.8 (1.6~5.8) 0.041 2.4 (1.1~5.3) 无 13(28.9) 48(53.3) 1.0 1.0 上学交通工具 私家车 8(17.8) 42(46.7) 0.264 1.0 校车 22(48.9) 16(17.8) 1.5 (0.8~2.1) 步行 15(33.3) 32(35.5) 1.0 每日洗手频率 高 11(24.4) 27(30.0) 0.427 0.6 (0.2~2.2) 一般 28(62.2) 52(57.8) 1.0 低 6(13.4) 11(12.2) 1.0 免疫接种史 1剂次 32(71.1) 79(87.7) < 0.001 0.2 (0.1~0.5) < 0.001 0.2 (0.1~0.4) 无免疫史 13(28.9) 11(12.3) 1.0 1.0 注:a条件Logistic回归分析中的P值。 2.4 突破病例的危险因素分析
将突破病例与对照组中有水痘疫苗接种史者相比较,比较其免疫接种年限、初次免疫年龄、年龄、性别等特征,结果显示:初次免疫<15月龄,疫苗接种年限>5年是发生突破病例的潜在危险因素。见表 3。
表 3 水痘突破病例潜在危险因素的单变量分析[n(%)]Table 3. Analysis of potential risk factors for varicella breakthrough cases[n(%)]变量 突破病例 对照组有免疫史者 χ2/u值 P值 年龄[月,M(P25, P75)] 9.2(7.5, 12.5) 9.3(6.8, 12.3) 11.23 0.735a 性别 0.03 0.861b 男 18(56.2) 43(54.4) 女 14(43.8) 36(45.6) 初次免疫年龄(月) 4.23 0.041b <15 19(59.4) 30(37.9) ≥15 13(40.6) 49(62.1) 接种年限(年) 4.06 0.044b ≤5 14(43.7) 51(64.6) >5 18(56.3) 28(35.4) 注: aWilcoxon rank-u检验;bPearson's chi-square检验。 2.5 1剂次水痘疫苗的保护效果分析
1剂次水痘疫苗的疫苗保护效果(vaccine effectiveness, VE)为77.9%(95% CI: 53.3%~92.1%),初次免疫年龄≥15月龄的保护效果为77.1%(37.1%~91.7%)。接种年限>5年的保护效果,低于<5年者(χ2=8.79,P=0.003)。见表 4。
表 4 水痘疫苗的保护效果分析[n(%)]Table 4. Analysis of the protective effectiveness of varicella vaccine[n(%)]病例 对照 VE(95% CI)a(%) P值 总体疫苗VE 有免疫史 32(71.1) 79(87.7) 77.9 (53.3~92.1) < 0.001 无免疫史 13(28.9) 11(12.3) Ref b 不同免疫时间VE(月) < 15 19(42.2) 31(34.5) 48.2(0.0~80.7) 0.189 ≥15 13(28.9) 48(53.3) 77.1(37.1~91.7) 0.003 无免疫史 13(28.9) 11(12.3) Ref b 不同接种年限VE(年) ≤5 14(31.1) 58(64.4) 79.6(44.9~92.5) 0.002 > 5 18(40.0) 21(23.3) 27.5(0.0~73.9) 0.536 无免疫史 13(28.9) 11(12.3) Ref b 注:aVE: (1-OR)×100%;bRef为参照水平(疫苗保护效果)。 3. 讨论
水痘是一种急性呼吸道传染病,主要易感者集中在托幼机构和中小学等集体机构。根据国家传染病公报,2015-2018年期间,水痘突发公共卫生事件和报告发病数逐年增多,已成为传染病防治和学校卫生工作的突出问题[3]。尽管目前已有较多关于水痘暴发疫情的报道,但在水痘疫苗高覆盖率下针对水痘传播因素和突破病例的潜在危险因素研究仍较少。本研究通过对2018年发生在江苏省中部地区一乡镇小学(水痘疫苗接种率达82%)的一起水痘暴发疫情开展了1 ∶ 2配对流行病学研究,对水痘的流行因素、1剂次水痘疫苗保护效果进行了系统分析。
本研究发现,45例水痘感染者中突破病例占比71.1%(95% CI:55.7~83.6),高于北京市两起暴发调查[4-5]。北京地区已经将1剂次水痘疫苗进行常规接种,但在高水痘疫苗覆盖率的中小学,水痘暴发疫情仍有发生[6]。相关研究显示,2剂次水痘疫苗接种程序可显著减少水痘突破病例的发生[7]。本研究中,水痘突破病例虽然占比很高,但是重症水痘病例占比很少,进一步说明水痘疫苗可以有效防止重症水痘。这与国内外相关研究报道相一致[8-9]。德国[10]、英格兰[11]的研究表明,虽然单个水痘病例的经济负担不高,但水痘发病率高,全人群的总的经济负担不容忽视。北京市每起小学水痘暴发疫情的总控制成本约为190 263元。突发事件给疫情控制机构带来的社会经济负担远超过给个人带来的社会经济负担。
本研究结果显示,参加课外辅导机构、有兄弟姐妹、有与水痘患者接触史、无水痘减毒活疫苗(varicella attenuated live vaccine, VarV)接种史是水痘传播的危险因素,这些因素间接说明了集体单位生活的儿童群体是水痘发病的高危人群[12]。学校在发现水痘病例后,应严格做好隔离,避免水痘暴发疫情的蔓延。从全球看,水痘疫苗免疫策略主要有1剂次和2剂次两种策略。报道显示1剂次VarV保护效果为86.3%(85.3%~87.3%),2剂次VarV的保护效果为97.3%(97.0%~97.6%)[13]。1剂次水痘疫苗可降低水痘发病率和死亡率,但不能预防局部病毒循环和暴发,接种2剂次水痘疫苗则可有效阻断水痘病毒传染[14]。本次研究显示1剂次VarV总体保护效果为77.9%(53.3~92.1),与相关研究结果一致[15]。本研究发现初次免疫年龄<15月龄的疫苗保护效果会降低,可以作为水痘疫苗免疫程序优化研究的参考。
-
表 1 突破病例与无免疫史病例的临床症状比较[n(%)]
Table 1. Comparison of clinical symptoms between breakthrough cases and non-immune cases[n(%)]
特征 突破病例 无免疫史病例 χ2值 P值 发热 5.47 0.019b 是 10(31.3) 9(69.2) 否 22(68.7) 4(30.8) 皮疹严重程度(个) 7.79 0.020b <50 25(78.1) 5(38.5) 50~ 6(18.8) 5(38.5) >500 1(3.1) 3(23.0) 并发症a 0.95 0.329 有 2(2.22) 2(6.7) 无 30(97.8) 11(93.3) 出疹部位(个) - 0.021b 1~ 14(43.8) 5(38.5) 3~ 16(50.0) 3(23.0) ≥5 2(6.2) 5(38.5) 病程(d) - <0.001b ≤10 23(73.3) 2(36.7) >10 9(26.7) 11(63.3) 注:a并发症包括肺炎、脑炎、皮肤继发细菌感染等疾病;bFisher确切概率法。 表 2 水痘暴发的流行因素分析[n(%)]
Table 2. Analysis of risk factors for Varicella transmission factors[n(%)]
特征 病例 对照 P值 单因素分析OR (95% CI)值 P值a 条件Logistic回归OR(95% CI)值 性别 男 25(55.6) 49(54.4) 0.903 0.9 (0.5~1.9) 女 20(44.4) 41(35.6) 1.0 是否有兄弟姐妹 是 28(62.2) 38(42.2) 0.030 2.3 (1.1~4.6) 0.037 2.5 (2.1~4.3) 否 17(37.8) 52(57.8) 1.0 1.0 参加课外辅导机构 是 29(64.4) 39(43.3) 0.021 2.4 (1.1~4.9) 0.019 2.6 (1.9~3.2) 否 16(35.6) 51(56.7) 1.0 1.0 与水痘患者接触史 有 32(71.1) 42(46.7) 0.029 2.8 (1.6~5.8) 0.041 2.4 (1.1~5.3) 无 13(28.9) 48(53.3) 1.0 1.0 上学交通工具 私家车 8(17.8) 42(46.7) 0.264 1.0 校车 22(48.9) 16(17.8) 1.5 (0.8~2.1) 步行 15(33.3) 32(35.5) 1.0 每日洗手频率 高 11(24.4) 27(30.0) 0.427 0.6 (0.2~2.2) 一般 28(62.2) 52(57.8) 1.0 低 6(13.4) 11(12.2) 1.0 免疫接种史 1剂次 32(71.1) 79(87.7) < 0.001 0.2 (0.1~0.5) < 0.001 0.2 (0.1~0.4) 无免疫史 13(28.9) 11(12.3) 1.0 1.0 注:a条件Logistic回归分析中的P值。 表 3 水痘突破病例潜在危险因素的单变量分析[n(%)]
Table 3. Analysis of potential risk factors for varicella breakthrough cases[n(%)]
变量 突破病例 对照组有免疫史者 χ2/u值 P值 年龄[月,M(P25, P75)] 9.2(7.5, 12.5) 9.3(6.8, 12.3) 11.23 0.735a 性别 0.03 0.861b 男 18(56.2) 43(54.4) 女 14(43.8) 36(45.6) 初次免疫年龄(月) 4.23 0.041b <15 19(59.4) 30(37.9) ≥15 13(40.6) 49(62.1) 接种年限(年) 4.06 0.044b ≤5 14(43.7) 51(64.6) >5 18(56.3) 28(35.4) 注: aWilcoxon rank-u检验;bPearson's chi-square检验。 表 4 水痘疫苗的保护效果分析[n(%)]
Table 4. Analysis of the protective effectiveness of varicella vaccine[n(%)]
病例 对照 VE(95% CI)a(%) P值 总体疫苗VE 有免疫史 32(71.1) 79(87.7) 77.9 (53.3~92.1) < 0.001 无免疫史 13(28.9) 11(12.3) Ref b 不同免疫时间VE(月) < 15 19(42.2) 31(34.5) 48.2(0.0~80.7) 0.189 ≥15 13(28.9) 48(53.3) 77.1(37.1~91.7) 0.003 无免疫史 13(28.9) 11(12.3) Ref b 不同接种年限VE(年) ≤5 14(31.1) 58(64.4) 79.6(44.9~92.5) 0.002 > 5 18(40.0) 21(23.3) 27.5(0.0~73.9) 0.536 无免疫史 13(28.9) 11(12.3) Ref b 注:aVE: (1-OR)×100%;bRef为参照水平(疫苗保护效果)。 -
[1] Suo L, Lu L, Wang Q, et al. Varicella outbreak in a highly-vaccinated school population in Beijing, China during the voluntary two-dose era[J]. Vaccine, 2017, 35(34): 4368-4373. DOI: 10.1016/j.vaccine.2017.06.065. [2] 麦冰, 陈抒豪, 黄劲梅, 等. 水痘疫苗在学校暴发疫情中保护效果的回顾性队列研究[J]. 微生物学免疫学进展, 2013, 41(1): 20-22. DOI: 10.13309/j.cnki.pmi.2013.01.007.Mai B, Cheng SH, Huang JM, et al. A retrospective cohort study on the effetiveness of varicella vaccine against varicella outbreak in school[J]. Prog in Microbiol Immunol, 2013, 41(1): 20-22. DOI: 10.13309/j.cnki.pmi.2013.01.007. [3] Michalik DE, Steinberg SP, Larussa PS, et al. Primary vaccine failure after 1 dose of varicella vaccine in healthy children[J]. J Infect Dis, 2008, 197(7): 944-949. DOI: 10.1086/529043. [4] Fu J, Wang J, Jiang C, et al. Outbreak of varicella in a highly vaccinated preschool population[J]. Int J Infect Dis, 2015, 37(1): 14-18. DOI: 10.1016/j.ijid.2015.06.003. [5] 王青海, 王旭, 刘淑岭, 等. 北京市西城区一起学校水痘突发公共卫生事件的调查分析[J]. 中国病毒病杂志, 2017, 7(5): 391-393. DOI: 10.16505/j.2095-0136.2017.05.016.Wang QH, Wang X, Liu L, et al. Investigation of varicella out breakthough cases in a primary school of Xicheng district[J]. Chin J Viral Dis, 2017, 7(5): 391-393. DOI: 10.16505/j.2095-0136.2017.05.016. [6] Wu QS, Liu JY, Wang X, et al. Effectiveness of varicella vaccine as post-exposure prophylaxis during a varicella outbreak in Shanghai, China[J]. Int J Infect Dis, 2018, 66(2): 51-55. DOI: 10.1016/j.ijid.2017.10.016. [7] Smith-Norowitz TA, Saadia TA, Norowitz KB, et al. Negative IgG Varicella Zoster Virus Antibody Status: Immune Responses Pre and Post Re-immunization[J]. Infect Dis Ther, 2018, 7(1): 175-181. DOI: 10.1007/s40121-017-0182-x. [8] Fu C, Wang M, Liang J, et al. The effectiveness of varicella vaccine in China[J]. Pediatr Infect Dis J, 2010, 29(8): 690-693. DOI: 10.1097/INF.0b013e3181d7380e. [9] Shady I. Seroprevalence of antibodies against varicella zoster virus and rubella virus among newly recruited expatriate healthcare workers: a cross-sectional study[J]. BMJ Open, 2018, 8(3): e019339. DOI: 10.1136/bmjopen-2017-019339. [10] Banz K, Wagenpfeil S, Neiss A, et al. The Burden of Varicella in Germany: Potential Risks and Economic Impact[J]. Eur J Health Econ, 2004, 5(1): 46-53. DOI: 10.1007/s10198-003-0200-7. [11] Hobbelen PH, Stowe J, Amirthalingam G, et al. The burden of hospitalisation for varicella and herpes zoster in England from 2004 to 2013[J]. J Infect, 2016, 73(3): 241. DOI: 10.1016/j.jinf.2016.05.008. [12] Vaidya SR, Tilavat SM, Kumbhar NS, et al. Chickenpox outbreak in a tribal and industrial zone from the Union Territory of Dadra and Nagar Haveli, India[J]. Epidemiol infect, 2018, 146(4): 476-480. DOI: 10.1017/S0950268818000201. [13] Pe? a Blasco G, Blasco Pérez-Aramendía MJ. A cost-benefit analysis of varicella vaccination in Aragon[J]. Arch Argent Pediatr, 2017, 115(5): 432-438. DOI: 10.5546/aap.2017.eng.432. [14] Ludwig B, Kraus FB, Allwinn R, et al. Loss of varicella zoster virus antibodies despite detectable cell mediated immunity after vaccination[J]. Infection, 2006, 34(4): 222-226. DOI: 10.1007/s15010-006-5616-9. [15] Leung J, Broder KR, Marin M. Severe varicella in persons vaccinated with varicella vaccine(breakthrough varicella): a systematic literature review[J]. Expert Rev Vaccines, 2017, 16(4): 391-400. DOI: 10.1080/14760584.2017.1294069. 期刊类型引用(36)
1. 徐先起,梅丽娟. 2021—2023年鄄城县水痘流行特征分析. 中外医学研究. 2025(02): 154-159 .  百度学术
百度学术2. 许勤勤,黄振水,孟希,李淑玲. 2005—2021年淄博市水痘流行病学特征及1~17岁人群水痘疫苗接种率分析. 微生物学免疫学进展. 2025(01): 67-72 .  百度学术
百度学术3. 李思杰,王跃会,邵宇,曹蕾,郑晖,陈磊. 2008—2022年重庆市渝中区疫苗针对法定呼吸道传染病流行趋势分析. 医学动物防制. 2024(08): 774-778+783 .  百度学术
百度学术4. 张永强,吉文博,蒙泽环,张仲凯. 白银市会宁县某小学一起水痘暴发疫情流行病学调查分析. 疾病预防控制通报. 2024(04): 76-78+92 .  百度学术
百度学术5. 陈蕊. 福安市2011—2023年水痘流行病学特征分析. 海峡预防医学杂志. 2024(03): 35-38 .  百度学术
百度学术6. 杨连建,杨长绢,段清浩. 重庆沙坪坝区2019—2023年水痘突破病例的流行特征分析. 现代预防医学. 2024(20): 3677-3681 .  百度学术
百度学术7. 张猛,丁薇薇,张雨晴. 2017—2022年无锡市滨湖区水痘流行病学特征分析. 中国校医. 2024(08): 603-605+634 .  百度学术
百度学术8. 梁夏楠,梁长威,徐斌,阳世雄,梁晓云,潘利花,谢艺红. 2014―2023年南宁市小学水痘突发公共卫生事件流行病学特征及相关因素. 中华疾病控制杂志. 2024(11): 1287-1294 .  本站查看
本站查看9. 王宝龙,赵秀娟. 水痘疫苗对儿童突破病例保护情况调查分析. 名医. 2024(19): 48-50 .  百度学术
百度学术10. 张小娟,权力,敖建军,华瑞珏,黄瑾. 2017—2019年上海市静安区水痘流行病学特征及疫苗接种情况分析. 上海预防医学. 2023(02): 122-125 .  百度学术
百度学术11. 尉雨佳,沙征,周蔓. 2016-2020年北京市西城区小学生水痘突破性感染病例分析. 首都公共卫生. 2023(02): 84-87 .  百度学术
百度学术12. 张莉,王荣会,何成丹. 重庆市巫山县2018—2022年水痘流行病学特征分析. 海峡预防医学杂志. 2023(03): 27-29 .  百度学术
百度学术13. 张荣瑜,蒋智,高岚. 贵阳市2010—2022年水痘流行病学特征分析. 现代预防医学. 2023(23): 4386-4391 .  百度学术
百度学术14. 李琦,谭小柏,张扬,张丽娟,吴艳玲,玄立印,王波,池红井,杨玉香,吕静,魏征. 2006年至2021年承德市水痘突发公共卫生事件流行特征和疫情处置分析. 河北医学. 2023(12): 2101-2105 .  百度学术
百度学术15. 黄恩妙,王翠玲. 水痘疫苗在学校水痘疫情中的保护效力分析. 河南预防医学杂志. 2022(03): 171-173+183 .  百度学术
百度学术16. 路淋. 2019年丹东市适龄儿童水痘疫苗接种情况分析. 预防医学论坛. 2022(02): 140-142 .  百度学术
百度学术17. 刘丽,汤奋扬,汪志国,于静,张磊,胡冉,高君,康国栋. 2021年江苏省预防接种综合服务管理信息系统学龄前儿童水痘疫苗接种率评价. 中国疫苗和免疫. 2022(03): 346-349 .  百度学术
百度学术18. 何左,周舟,李庆棠. 2004-2021年大理白族自治州水痘突发公共卫生事件流行特征分析. 中华灾害救援医学. 2022(06): 309-313 .  百度学术
百度学术19. 陈宏标,古子豪,周小峰,罗经伟,彭伟军,刘丽珍. 广东省深圳市某区一起水痘暴发疫情调查和基于SEIR模型对疫情防控效果的评估. 疾病监测. 2022(06): 855-860 .  百度学术
百度学术20. 舒哲,赵文柳,周奕丞,武英. 突破性水痘患者临床表现及危险因素. 现代养生. 2022(21): 1811-1814 .  百度学术
百度学术21. 佟鑫. 关于南京市某区幼儿园一起水痘暴发疫情的调查分析. 齐齐哈尔医学院学报. 2022(17): 1664-1667 .  百度学术
百度学术22. 王翠玲,吕海英,李雷,吴雯,蔡乾春,陈小红. 水痘疫苗在学校水痘暴发疫情中保护效果的病例对照研究. 现代预防医学. 2022(23): 4390-4393 .  百度学术
百度学术23. 杨英,刘俊,汪振娟,傅钰,郭黄吉. 遵义市1起小学暴发疫情水痘突破病例的临床特征和危险因素. 中国疫苗和免疫. 2021(01): 98-101 .  百度学术
百度学术24. 徐蕊,葛为民. 平顶山市2006—2019年学校水痘突发公共卫生事件流行病学特征及应急处置分析. 医药论坛杂志. 2021(06): 63-66+129 .  百度学术
百度学术25. 张嘉陵,谷利妞,孙莉莉,潘佑记. 连云港市35起水痘突发公共卫生事件中突破病例流行病学特征分析. 实用预防医学. 2021(06): 728-730 .  百度学术
百度学术26. 韦淑,李晓琼,吴潇潇,黄振博,吴彩艳,聂志明,卢洁. 南宁市良庆区0~14岁儿童水痘流行特征及疫苗接种情况分析. 应用预防医学. 2021(03): 255-256 .  百度学术
百度学术27. 韦忠信,罗彩婵,陈艳,黄永红. 2011~2020年田东县水痘流行特征分析. 右江医学. 2021(06): 456-459 .  百度学术
百度学术28. 丁旭,任达飞,高前荣,宁乐婷,肖艳芳. 2010—2020年铜仁市水痘流行病学特征分析. 现代预防医学. 2021(18): 3411-3414 .  百度学术
百度学术29. 周路平,石晓娟,周洋,张颖,周莉薇. 宁夏3~5岁幼托儿童水痘疫苗接种率及影响因素调查. 现代预防医学. 2020(02): 336-339+362 .  百度学术
百度学术30. 徐志荣,郭鸿平,周婷,黄华,郭滟萍,李琼芬. 昆明市官渡区2019年1-14岁健康儿童水痘-带状疱疹病毒抗体水平. 中国疫苗和免疫. 2020(03): 290-292 .  百度学术
百度学术31. 赵寒,李勤,杨琳,夏宇,宿昆. 重庆市2014-2018年学校突发公共卫生事件流行特征分析. 重庆医学. 2020(13): 2201-2205 .  百度学术
百度学术32. 蒋春梅. 2010-2018年徐州市水痘流行特征分析. 现代预防医学. 2020(13): 2328-2331+2341 .  百度学术
百度学术33. 方芳芳,丁佳琳,殷明,宋沛云,周全斌. 2009-2018年无锡市荣巷街道水痘流行特征分析. 医学动物防制. 2020(11): 1034-1037 .  百度学术
百度学术34. 杨欣,赵艳艳,佘慧中,张军. 肥西县2014-2018年水痘流行特征分析. 安徽预防医学杂志. 2020(05): 380-382+405 .  百度学术
百度学术35. 杨月,王旭阳,韩一楠,林茜. 大连市某小学一起水痘暴发流行特征调查分析. 社区医学杂志. 2019(21): 1331-1334 .  百度学术
百度学术36. 王翠玲,黄莉莉,陈小红,黄恩妙. 中山市2013-2018年水痘突发公共卫生事件特征和水痘疫苗保护效果. 中国疫苗和免疫. 2019(06): 635-638 .  百度学术
百度学术其他类型引用(1)
-






 下载:
下载:
 下载:
下载: